Regardless of the name, new capacitors such as ultracapacitors or supercapacitors are much larger than those of conventional capacitors. Directly speaking, you can now purchase a radial leaded on-board capacitor with a rating of 5~10F/2.5V, a flash-cell capacitor with a rating of 120~150F/5V, and a larger single capacitor can reach 650. ~3000F/2.7V capacitance value. Note that the capacitance values ​​of all these capacitors are in Farads. Not too long ago, two thousand microfarads were considered large capacitors. If you need more types of capacitors, you can order a variety of capacitors with capacitor ratings from 20F to 500F and voltage ratings from 15V to 390V. If you use the appropriate serial/parallel combination, you can even drive a bus with this type of capacitor—yes, not the wiring on the board, but the passenger bus. (Although hybrid fuel systems, chemical batteries and fuel cells are just around the corner, they have not been officially put into use). When developing ultracapacitors, people did not find any new laws of physics. In fact, the principle of supercapacitance still goes back to the German physicist Helmholtz. Like ordinary capacitors, supercapacitors store energy in the form of stored charges between two "plates." The magnitude of the capacitance is proportional to the area of ​​the plates and the dielectric material used between the plates, and inversely proportional to the distance between the plates. However, the principle of supercapacitors is different. Before we realized the huge capacitance with supercapacitors, we have mastered the principle of electrolytic chemistry (electrolyTIcs). Ultracapacitors are not electrolytic chemistry, but understanding electrochemistry helps us understand the new technology of supercapacitors. It is called electrolytic chemistry because one (or two) of its "plates" are non-metal electrolytes formed on the surface of a metal substrate. During the manufacturing process, a voltage drive current flows from the anode metal plate through the conductive plating bath to the cathode. This creates an insulating metal oxide-dielectric on the surface of the anode. In electrolytic chemistry, when the electrode is immersed in the electrolytic solution, charge accumulation and charge separation occur at the electrode interface. The accumulation of reversely charged ions in the electrolyte compensates for the residual charge on the electrode surface. This interface is called the Helmholtz layer. The structure of the ultracapacitor is no longer the structure of a flat electrode (or a flattened flat electrode) that is filled with a dielectric material - like a peanut butter in the middle of a sandwich. In the supercapacitor, charge/discharge of charges occurs at the interface between the porous carbonaceous material or the porous metal oxide in the electrolyte. The Helmholtz layer causes an effect called a double layer capacitor. When a DC voltage is applied to both ends of the porous carbon electrode in the supercapacitor, the cation or anion for charge compensation accumulates in the electrolyte around the charged electrode. If electron migration does not occur at the interface, the "two-layer" separated charge (electrons or electron holes on the metal side, and the cation or anion on the electrolyte side of the interface boundary) will appear on the interface (see figure 1)). Figure 1: The supercapacitor essentially consists of two plates and a separator suspended in an electrolyte. The positive electrode plate attracts anions in the electrolyte. The negative plate attracts cations. This forms a so-called electrochemical double layer capacitor (EDLC) with a two-layer capacitive storage structure. The size of the Helmholtz-region capacitor depends on the area of ​​the porous carbon electrode and the ion capacity in the electrolyte. The capacitance per square centimeter on the double layer electrode is 10,000 times that of the ordinary dielectric capacitor. This is because the distance between the charges in the two-layer electrode is only about 0.3 to 0.5 nm, while in electrolytic chemistry this distance is 10 to 100 nm, and the mica or polystyrene capacitance is 1000 nm. We have already understood the principle of this "double layer" electrode. However, this two-layer structure reduces the theoretical capacitance that the actual device should achieve because the supercapacitor includes a pair of electrodes, each of which has an area of ​​only half of the total area. In addition, the supercapacitor is actually a series of two capacitors connected in series. Therefore, the actual capacitance of the supercapacitor is only a quarter of the theoretical capacitance calculated from the electrode area and ion capacity. If you want to learn more about the theory of supercapacitors, you can read an article entitled "Electrochemical Capacitors, Their Nature, FuncTIon, and ApplicaTIons" from the Electrochemistry Encyclopedia. The author is Ottawa University. Brian E. Conway of the Department of Chemistry. Conway has been researching ultracapacitor theory for decades and has made important contributions to this area. Some literatures like to confuse batteries with supercapacitors, masking many important differences between the two: The battery stores the energy calculated in watt-hours, and the capacitance stores the power in watts. The battery provides electrical energy with a constant chemical reaction for a long period of time, and the charging time is relatively long, and the characteristics of the charging current are demanding. Instead, the charging of the capacitor is done by loading the voltage across it, and the charging speed is largely dependent on the external resistance. The battery is capable of outputting electrical energy at a substantially constant voltage for a longer period of time. The discharge speed of the capacitor is fast, and the output voltage is exponentially attenuated. High Voltage Linear Power Supplies HVLP series Linear High Voltage Power Supplies are High-voltage DC Power Supplies that achieve AC/DC conversion through power frequency transformers and transistor loop control. Compared with switching high voltage power supplies, linear high-voltage power supplies have higher stability, higher accuracy, and lower output ripple. And the most important, because of the use of the power frequency AC/DC conversion principle, the linear power supply has no high-frequency radiation interference, and it is especially suitable for use in places with restrictions on EMC and EMI.
High-voltage Linear Power Supplies,Linear High Voltage Power Supplies, HV Linear Power Supplies, Linear HV Power Supplies, Linear HVPS Yangzhou IdealTek Electronics Co., Ltd. , https://www.idealtekpower.com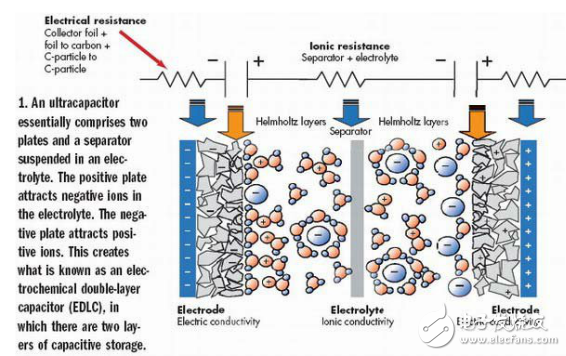

The whole series linear power supply adopts industrial-grade metal chassis, pure copper AC/DC multi-insulation high-voltage transformer with varnish treatment, high-reliability multi-transistor filter loop, ensuring the power supplies can run for a long time at full load with high stability, high accuracy, and ultra-low ripple electronic characteristics, equipped with a complete protection circuit, which can better ensure the reliability of the linear power supply itself and the safety of the customer's load.
The output voltage and current can be adjusted by the 10-turn potentiometer with scale and lock on the front, equipped with 4 1/2-digit high-resolution LED meters for output value reading, and RS communication interface can also be added for remote control and monitoring of linear power supplies.
This series of linear high voltage power supplies are mainly used for gas discharge, high-voltage electronic tubes, and can also be applied for other electronic components burn-in test.
Because the output this power supply has HV, the output MUST be connected to the chassis for fixed grounding to ensure the personal safety of the user.